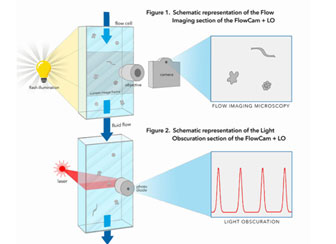
FlowCam® LO (Light Obscuration)
New FlowCam LO instrument combines our patented flow imaging microscopy technology with an embedded light obscuration particle counter to provide you with the necessary data for USP regulations and validations with images.
It can obtain LO data to meet regulatory guidelines, and also verify your data with real images, all with a single instrument, and a single sample run.
In the FlowCam LO, a single sample passes continuously through two flow cells.The first uses FlowCam technology to capture digital images and the second uses a light obscuration module to provide data necessary for USP compliance.
While analysis with light obscuration is standard, the FDA has long made clear that size data alone, collected with light obscuration, is not adequate to ensure safe and effective drugs, and that it's necessary to provide validation and imaging data using orthogonal methods. FlowCam LO has the orthogonal method built in.
FlowCam LO provides:
- USP <787> compliance data with light obscuration
- Flow imaging microscopy validation of particle types with images
- Identification of additional particles missed by light obscuration alone
- Greater understanding of drug product formulation
![]() The Importance of Images :
The Importance of Images :
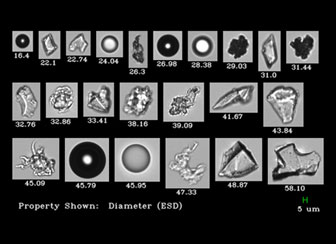
Given that Light Obscuration compares all particles to a sphere (equivalent spherical diameter or ESD), it's possible that extrinsic particles roughly the same size as your proteins would pass the LO USP size distribution test.
The image below shows the variety of particles you might see with a similar ESD (shown here: proteins, agglomerates, silicone droplets, air bubbles and other contaminants - silica, glass shards).A sample like this might pass USP size limits, but still fail at the trial stage. Verify your data with images, with the FlowCam LO.
FlowCam LO Specifications:
- Particle Size Range: 2 µm to 70 µm
- Magnification: 10X
- Flow Cells: Flow Imaging Module: 80 µm x 700 µm quartz, LO Module: 1 mm x 0.4 mm quartz
- Sample Processing Capability: 0.2 mL / minute
- Sample Volume Range: 250 µL - 2 mL
- Flow Imaging Module Camera: High resolution (1920 x 1200 pixels) CMOS (Monochrome and color available)
- Frame Rate: Shutters up to 100 frames per second
- LO Light Source: Solid-state laser diode, 785 nm
- LO Detection Method: Light extinction, volumetric
- Fluidics: Micro-syringe pump with 2.5 mL syringe
- Equipped with image analysis software VisualSpreadsheet®